Science
›
Chemistry
›
Theoretical Chemistry
›
Chemical Thermodynamics
Advertisement
-
-
-
-
States of Matter 107
-
Solutions 61
-
-
-
Nomenclature 15
-
Hydrocarbons 13
-
Alcohols 3
-
Amines 2
-
Polymers 5
-
-
-
Biomolecules 13
-
Proteins 26
-
Enzymes 4
-
Lipids 14
-
Metabolism 40
-
Vitamins 5
-
Hormones 1
-
-
-
-
-
-
-
-
-
-
Alloys 1
-
-
-
-
Nucleus 1
-
Cell Cycle 18
-
-
Genomics 1
-
-
-
Ecosystem 35
-
Biomes 24
-
Food Chains 15
-
Biodiversity 23
-
Habitat 4
-
-
-
Skeletal System 116
-
Nervous System 111
-
-
-
-
Porifera 6
-
Cnidaria 6
-
Nematoda 6
-
Annelida 7
-
Arthropoda 12
-
Mollusca 4
-
Chordata 5
-
Fish 76
-
Amphibians 10
-
Reptiles 14
-
Birds 2
-
Mammals 106
-
Anatomy 17
-
Physiology 20
-
Reproduction 23
-
Behavior 63
-
-
Bacteriology 33
-
Virology 33
-
Mycology 16
-
Parasitology 51
-
-
-
GMOs 4
-
-
Bio Enzymes 21
-
Bio Hormones 32
-
-
B Cells 1
-
-
-
-
Clouds 1
-
-
Marine Life 47
-
-
-
-
-
Wetlands 5
-
-
Tsunamis 1
-
-
11th Grade Chemical Thermodynamics Quizzes, Questions & Answers
Think your child knows their 11th grade Chemical Thermodynamics? Read more
Challenge them with our fun and engaging Chemical Thermodynamics quizzes! Perfect for reinforcing classroom learning and discovering new facts about the world around them.
Read less
Top Trending Chemical Thermodynamics Quizzes
Dive into our Chemistry Quiz designed to test your knowledge on thermodynamics, including concepts like heat transfer, entropy, and electrochemistry. This quiz assesses key skills in understanding energy changes in chemical...
Questions: 20 | Attempts: 1003 | Last updated: Mar 20, 2025
-
Sample QuestionWhich of the following relations is correct?




Embark on a journey through the realm of heat and energy transformations with our "Thermochemistry Quiz: Mastering Heat in Chemistry." This quiz is designed to challenge and deepen your understanding of the principles...
Questions: 15 | Attempts: 492 | Last updated: Oct 22, 2025
-
Sample QuestionWhich of the following is NOT an example of an energy transformation?




Created by
Dr. K. S. Prakash
Assistant Professor of Chemistry
Bharathidasan Govt. College for Women (Autonomous)
Puducherry.
Questions: 10 | Attempts: 102 | Last updated: Mar 21, 2025
-
Sample QuestionThe first law of thermodynamics is basically the same as which law from physics?




What do you know about bond energy? Bond energy is the measure of a chemical bond to strengthen a chemical bond. This boost means that it demonstrates bond energy tells us how likely a pair of atoms is to remain bonded in the...
Questions: 18 | Attempts: 501 | Last updated: Mar 21, 2025
-
Sample Question∆H°f stands for the ……………




Welcome to the "Hess's Law Essentials: Constant Heat Summation Quiz," where you'll embark on a journey into the world of thermodynamics and chemical reactions. Hess's Law is a fundamental principle that...
Questions: 10 | Attempts: 64 | Last updated: Oct 22, 2025
-
Sample QuestionWhat is the enthalpy change for a reaction if it can be expressed as the sum of two other reactions?




Popular Chemical Thermodynamics Quizzes
Endothermic and Exothermic are chemical reactions that absorb and release heat. An example of an endothermic reaction is photosynthesis. An exemplar of an exothermic reaction is combustion. Specifically, exothermic reactions...
Questions: 10 | Attempts: 6428 | Last updated: Oct 28, 2025
-
Sample QuestionC6H12O6 + 6O2 à 6CO2 + 6H2O ∆H = -2803 kJ


The 'Thermochemistry Review' assesses understanding of key concepts such as enthalpy, energy units, and heat transfer calculations. It evaluates practical application through problem-solving, relevant for students or...
Questions: 16 | Attempts: 5172 | Last updated: Sep 12, 2025
-
Sample QuestionWhat do we call the heat content of a system




Get ready for this amazing Chemistry Heat and Temperature Quiz. It takes into consideration the resultant heat and temperatures of different reactions and elements. How good are you with the topic? Take the quiz and find out....
Questions: 10 | Attempts: 4443 | Last updated: Mar 22, 2025
-
Sample QuestionHow many joules of heat is required to heat 68.00 grams of aluminum foil from 55.00 °C to 93.00 °C in case aluminum has a specific heat of 0.90 J/g °C?




Do you know about lattice energy and Born Haber Cycle? Can you attempt this MCQ quiz on lattice energy and Born Haber Cycle? Give this quiz a try and check your knowledge of these two concepts. The Born–Haber cycle is a...
Questions: 10 | Attempts: 3300 | Last updated: Aug 21, 2025
-
Sample QuestionWhich of the following compounds is expected to have the highest lattice energy?




Prepared by: G. KUPPUSWAMY, P.G.Asst., Pachaiyapp's HSS, Kanchipuram.
...
Questions: 9 | Attempts: 1420 | Last updated: Mar 21, 2025
-
Sample Question
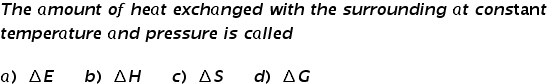




This quiz is to help students prepare for the Thermochemistry Exam
Questions: 11 | Attempts: 1295 | Last updated: Mar 21, 2025
-
Sample QuestionWhich of the following statements are correct?





Recent Chemical Thermodynamics Quizzes
Endothermic and Exothermic are chemical reactions that absorb and release heat. An example of an endothermic reaction is photosynthesis. An exemplar of an exothermic reaction is combustion. Specifically, exothermic reactions...
Questions: 10 | Attempts: 6428 | Last updated: Oct 28, 2025
-
Sample QuestionC6H12O6 + 6O2 à 6CO2 + 6H2O ∆H = -2803 kJ


The 'Thermochemistry Review' assesses understanding of key concepts such as enthalpy, energy units, and heat transfer calculations. It evaluates practical application through problem-solving, relevant for students or...
Questions: 16 | Attempts: 5172 | Last updated: Sep 12, 2025
-
Sample QuestionWhat do we call the heat content of a system




Get ready for this amazing Chemistry Heat and Temperature Quiz. It takes into consideration the resultant heat and temperatures of different reactions and elements. How good are you with the topic? Take the quiz and find...
Questions: 10 | Attempts: 4443 | Last updated: Mar 22, 2025
-
Sample QuestionHow many joules of heat is required to heat 68.00 grams of aluminum foil from 55.00 °C to 93.00 °C in case aluminum has a specific heat of 0.90 J/g °C?




Do you know about lattice energy and Born Haber Cycle? Can you attempt this MCQ quiz on lattice energy and Born Haber Cycle? Give this quiz a try and check your knowledge of these two concepts. The Born–Haber cycle is a...
Questions: 10 | Attempts: 3300 | Last updated: Aug 21, 2025
-
Sample QuestionWhich of the following compounds is expected to have the highest lattice energy?




Prepared by: G. KUPPUSWAMY, P.G.Asst., Pachaiyapp's HSS, Kanchipuram.
...
Questions: 9 | Attempts: 1420 | Last updated: Mar 21, 2025
-
Sample Question
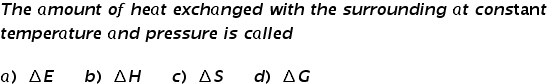




This quiz is to help students prepare for the Thermochemistry Exam
Questions: 11 | Attempts: 1295 | Last updated: Mar 21, 2025
-
Sample QuestionWhich of the following statements are correct?





Advertisement