What Is Dipeptides Definition, Formation, and Biological Roles? Understand Its Structure & Significance
Lesson Overview
- Definition and Structure of Dipeptides
- Peptide Bond Formation (Condensation of Two Amino Acids)
- Dipeptides in Protein Digestion and Absorption
- Examples of Biologically Important Dipeptides
- Neuroactive Dipeptides and Signaling Functions
- Dipeptides in Digestion and Taste
- Cyclic Dipeptides and Diketopiperazines
Dipeptides are organic compounds composed of two amino acids linked by a single peptide bond. They represent the simplest type of peptide, yet they play diverse roles in chemistry, nutrition, and physiology. Despite their small size, dipeptides can have significant biological functions – from being an artificial sweetener in our diet (as in the case of aspartame) to acting as signaling molecules in the body (such as the neuroactive dipeptide kyotorphin).
This lesson provides a detailed overview of dipeptides, explaining what they are, how they form, and their various roles in biological systems. Key concepts include how peptide bonds form, examples of important dipeptides, their involvement in digestion and cell signaling, and special types like cyclic dipeptides.
Definition and Structure of Dipeptides
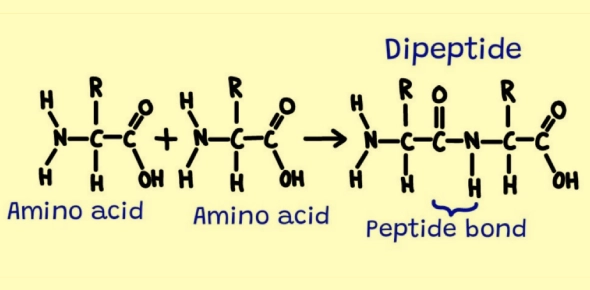
A dipeptide is defined as a molecule consisting of two amino acids joined by one peptide bond. In a dipeptide, one amino acid's carboxyl group is linked to the amino group of the second amino acid. This peptide bond (an amide linkage) holds the two amino acid residues together and results in a chain of two units. Important points about dipeptide structure include:
- Constituents: The two amino acids in a dipeptide can be the same or different. For example, glycylglycine (Gly–Gly) is the simplest dipeptide, made of two glycine molecules, whereas alanylserine (Ala–Ser) is a dipeptide composed of two different amino acids.
- N-terminus and C-terminus: A linear dipeptide has two distinct ends. The end with a free amino group (–NH₃⁺ at physiological pH) is called the N-terminal, and the end with a free carboxyl group (–COO⁻ at physiological pH) is the C-terminal. Conventionally, peptide sequences are written from the N-terminus to the C-terminus. This means that the order of the two amino acids matters: for instance, Ala–Ser and Ser–Ala are two different dipeptides (each has the same two amino acids but in reversed sequence).
- One Peptide Bond: The term dipeptide refers to the whole molecule and not the bond itself. There is only one peptide bond present in a dipeptide. (Sometimes students mistakenly say "dipeptide bond," but the correct term is simply peptide bond, since "di-" refers to the two amino acids.)
- General Structure: In its neutral form, a dipeptide can be represented as H₂N–CHR¹–CO–NH–CHR²–COOH (where R¹ and R² are the side chains of the two amino acids). This structure contains the –CO–NH– linkage of the peptide bond. The backbone of a dipeptide (ignoring side chains) is thus typically –NH₃⁺–CHR¹–CO–NH–CHR²–COO⁻ in zwitterionic form at physiological pH.
Peptide Bond Formation (Condensation of Two Amino Acids)
Peptide bond formation is a key chemical reaction that creates dipeptides from individual amino acids. When two amino acids combine, the carboxyl (-COOH) group of one amino acid reacts with the amino (-NH₂) group of the other. This process is a condensation (dehydration synthesis) reaction, meaning a molecule of water is released as the bond forms. Specifically, the OH from the carboxyl group of one amino acid and an H from the amino group of the other are removed to form H₂O, while the remaining fragments join to form an amide (peptide) bond:
- Energy and Enzymes: Forming a peptide bond is not spontaneous in aqueous solution – it requires an input of energy or catalytic help. In living organisms, peptide bonds (and thus dipeptides) are formed by enzymes or during protein synthesis on the ribosome, using activated amino acid intermediates. In the laboratory, coupling agents or other activating methods are used to synthesize dipeptides.
- Bond Characteristics: The peptide bond has partial double-bond character due to resonance, making it planar and relatively rigid. All four atoms connected by a peptide bond (the carbonyl carbon and oxygen, and the amide nitrogen and its attached hydrogen) lie in the same plane. This is a consideration in larger peptide structures, though in a dipeptide the molecule is very short.
- Orientation Matters: After the peptide bond forms, the dipeptide has a definite orientation (N-terminal on one end, C-terminal on the other). It's important to note that one cannot "flip" a dipeptide end-to-end without changing its identity – for example, glycine–alanine (Gly–Ala) vs alanine–glycine (Ala–Gly) are distinct molecules because the sequence of the amino acids is reversed.
(Table: Formation of a Dipeptide by Condensation)
| Reactants | Reaction Process | Products |
| Two amino acids (e.g. A, B) | Condensation (remove H₂O) | Dipeptide (A–B) + Water (H₂O) |
| –COOH of amino acid A + | → forms –CO–NH– (peptide bond) | (A–B is a dipeptide with a |
| –NH₂ of amino acid B | peptide bond linking A and B) |
Take This Quiz
Dipeptides in Protein Digestion and Absorption
Dipeptides frequently appear as intermediates during the digestion of proteins. When we eat proteins, enzymes in the digestive tract break the long polypeptide chains into smaller fragments. Many of these fragments are dipeptides (two-amino-acid pieces), which can then be further processed or absorbed. Key points regarding dipeptides in digestion include:
- Proteolysis to Dipeptides: Enzymes like pepsin in the stomach and trypsin and chymotrypsin in the small intestine cleave proteins into shorter chains. Subsequent enzymes called peptidases (including dipeptidases) can split these short chains. End-products of protein digestion are often a mix of free amino acids and small peptides, primarily dipeptides and tripeptides.
- Absorption: The cells lining the small intestine can efficiently absorb certain dipeptides directly. Specialized transporters (such as the PepT1 transporter) allow dipeptides and tripeptides to enter intestinal cells, where they are usually further broken down into amino acids. This is actually a faster route for nutrient uptake than absorbing amino acids one by one.
- Gastrin Secretion (G-cell Stimulation): A notable physiological response to the presence of peptides is the stimulation of G-cells in the stomach. G-cells are enteroendocrine cells that secrete the hormone gastrin. Dipeptides in the stomach lumen stimulate G-cells to release gastrin, especially during protein digestion. Gastrin then triggers the secretion of gastric acid by parietal cells.
- Breaking Down vs. Building Up: It's worth noting that in biological systems, dipeptides are more commonly produced by breaking down larger proteins (as just described) rather than by directly coupling free amino acids. While two free amino acids can chemically combine to form a dipeptide, in living organisms this typically happens as part of a larger protein assembly process or via specific dipeptide-synthesizing enzymes in microbes. In human metabolism, we mostly see dipeptides as transient breakdown products of dietary proteins.
Examples of Biologically Important Dipeptides
Numerous specific dipeptides have been identified, each composed of a unique pair of amino acids and often having a distinct role or occurrence. The table below highlights several notable examples of dipeptides, their amino acid composition, and their significance:
| Dipeptide Name | Composition (Amino Acids) | Notable Role or Occurrence |
| Aspartame | Aspartic acid + Phenylalanine (methyl ester) | Artificial sweetener used as a sugar substitute in foods and beverages. It is a modified dipeptide; upon digestion it breaks down into its two constituent amino acids. |
| Carnosine | Beta-alanine + Histidine | Found in high concentrations in skeletal muscle and brain tissue of mammals. Acts as a pH buffer and antioxidant in muscle; thought to help delay muscle fatigue. |
| Anserine | Beta-alanine + 1-methylhistidine | Present in muscle (especially in poultry and some mammals). Similar in function to carnosine; helps buffer pH in muscles. It is essentially a methylated form of carnosine. |
| Balenine (Ophidine) | Beta-alanine + Tau-methylhistidine | Found in muscles of various mammals (including humans) and birds. Related to carnosine/anserine family; its function is also linked to muscle metabolism and pH buffering. |
| Homoanserine | Homoserine + Alanine (dipeptide also described as N-(4-aminobutyryl)-histidine) | Identified in the brain and muscles of mammals. Belongs to the carnosine/anserine family of dipeptides. Its presence in both neural and muscle tissue suggests roles in neurotransmission and muscle function. (Homoserine is an amino acid analogue; in this dipeptide it pairs with alanine.) |
| Kyotorphin | Tyrosine + Arginine (L-Tyr-L-Arg) | A neuroactive dipeptide found in the brain (and also detected in small amounts in other tissues like skeletal muscle). Kyotorphin acts as a neuromodulator, notably exhibiting analgesic (pain-relieving) effects by influencing pain pathways. |
| Glorin | Derived from Glutamic acid + Ornithine (modified residues) | A chemotactic signaling dipeptide in certain slime molds (e.g. Polysphondylium violaceum). It attracts slime mold cells, guiding their movement. Glorin is a specialized example of a dipeptide functioning as an extracellular signal in lower organisms. |
| Barettin (cyclic) | Cyclo-(6-bromo-tryptophan – arginine) | A cyclic dipeptide (diketopiperazine) isolated from a marine sponge (Geodia barretti). Contains a brominated tryptophan. It has been studied for potential biological activities (e.g., antifouling or anticancer properties). This is an example of a modified dipeptide in nature. |
Neuroactive Dipeptides and Signaling Functions
While most neurotransmitters are single amino acids or larger peptides, there are dipeptides that have notable signaling roles in the nervous system. Kyotorphin is a prime example of a neuroactive dipeptide. Chemically known as L-tyrosyl-L-arginine, kyotorphin is produced in certain neurons.
It does not interact with opioid receptors directly, but it can induce analgesia (pain relief) by causing the release of enkephalins (which are endogenous opioid peptides). Thus, kyotorphin participates in pain modulation pathways in the brain. Research into kyotorphin has highlighted:
- Its presence in mammalian brain regions involved in pain perception.
- The fact that as a dipeptide it can cross some biological barriers more easily than larger peptides, potentially making it interesting for drug development.
- Kyotorphin's role is specialized; it's not a widespread neurotransmitter, but it demonstrates that even very small peptides like dipeptides can have specific and significant neuromodulatory effects.
In addition to kyotorphin, dipeptides like carnosine (beta-alanyl-L-histidine) might also influence neural function. Carnosine is abundant in the brain and is thought to act as an antioxidant and neuroprotective agent, scavenging free radicals and chelating metal ions. Though carnosine's role is more metabolic/protective than signaling, its high concentration in brain tissue underlines that dipeptides are integral to neural chemistry.
Finally, outside the human nervous system, some dipeptides serve as signals in other organisms. For instance, glorin (mentioned above) is used by slime mold cells to communicate and aggregate. Even in bacteria and immune systems, small peptides (including dipeptides or tripeptides) can serve as chemoattractants or signaling molecules.
Dipeptides in Digestion and Taste
Beyond their role in stimulating gastrin release (via G-cells in the stomach, as described earlier), dipeptides can also contribute to the sensory experience of foods. Some dipeptides have flavors or taste-modulating properties. For example, the dipeptide aspartame is widely used as a high-intensity sweetener. Aspartame's sweet taste comes from its specific amino acid combination (aspartic acid linked to phenylalanine methyl ester). It is approximately 200 times sweeter than table sugar on a weight basis.
When consumed, aspartame is metabolized into its constituent amino acids (and a small amount of methanol from the methyl ester), which are then handled by the body's normal amino acid pathways. Notably, because aspartame contains phenylalanine, individuals with the genetic condition phenylketonuria (PKU) must monitor their intake of this dipeptide sweetener.
Additionally, protein hydrolysates (partially broken-down proteins rich in dipeptides and tripeptides) often have a savory taste (umami) due to the presence of small peptides. In culinary science, the flavor of broths or aged foods is partly due to short peptides produced from protein breakdown. Certain dipeptides may contribute to these taste properties or act as taste enhancers. While individual free amino acids (like glutamate in MSG) are known for flavor, the combined effect of dipeptides is an area of interest in food chemistry.
Cyclic Dipeptides and Diketopiperazines
Not all dipeptides are linear chains; some exist in cyclic form. When a dipeptide's N-terminus and C-terminus link together, a cyclic dipeptide is formed. These cyclic dipeptides are also known as 2,5-diketopiperazines (abbreviated DKPs) because the ring contains two ketone (=O) and two amide (NH) functionalities in a six-membered ring (a structure typical of cyclo-dipeptides). Key aspects of cyclic dipeptides include:
- Formation: Diketopiperazines can form spontaneously from linear dipeptides under certain conditions. If a dipeptide's amino and carboxyl ends react with each other (in a dehydration reaction analogous to peptide bond formation), the molecule cyclizes into a ring structure. This often happens when dipeptides are heated, exposed to certain chemical conditions, or even sometimes during storage of peptide solutions.
- Peptide Synthesis By-products: In synthetic organic chemistry and biochemistry labs, cyclic dipeptides are notorious as side products in peptide synthesis. For example, during the chemical synthesis of longer peptides, if the N-terminus of a growing peptide and the activated carboxyl group of its second residue react internally, they can detach as a cyclized dipeptide (a diketopiperazine), especially when attempting to couple only two amino acids. This undesired cyclization reduces the yield of the intended peptide product. Peptide chemists take steps to minimize this by-product (such as using protecting groups and optimized reaction conditions) because diketopiperazine formation can compete with the desired peptide bond formation.
- Natural Occurrence: Many diketopiperazines have been found in nature. They can be produced by microbes, fungi, marine organisms, and even as degradation products of larger peptides. Some cyclic dipeptides have interesting biological activities. For instance, barettin, a brominated cyclic dipeptide from a marine sponge, has been studied for its antifouling properties (it prevents barnacles from settling, for example). Other cyclic dipeptides (some from bacteria or fungi) exhibit antibiotic, antiviral, or anticancer activities, making them of interest in drug discovery research.
- Special Properties: Cyclization often makes a dipeptide more rigid and sometimes more stable to enzyme degradation than the linear form. This can enhance bioactivity or allow them to fit into biological receptors differently. The cyclic structure also eliminates the distinct N- and C- termini, so these molecules don't carry the typical zwitterionic ends of linear peptides. Instead, they exist solely as a closed ring.
Take This Quiz
Rate this lesson:
 Back to top
Back to top