Metabolism and Enzymes An Easy Guide: A Complete Guide with Real-World Relevance
Lesson Overview
- What Is Metabolism?
- Thermodynamics in Metabolism
- ATP: The Energy Currency
- Enzymes: Biological Catalysts
- Activation Energy Explained
- Factors Affecting Enzyme Activity
- Enzyme Inhibition and Regulation
- Feedback Inhibition
- Role of Cofactors and Coenzymes
- Allosteric Regulation
- Sample Concept Checks
- Enzyme Graph: With vs. Without Enzyme
- Misconceptions to Avoid
- Core Concepts
- Key Takeaway
Your body is a dynamic engine where chemical reactions keep you alive. These reactions need to be fast, accurate, and energy-efficient. This is where two core concepts come into play: metabolism, the total of all cellular chemical processes, and enzymes, the proteins that accelerate them. This lesson covers the core principles students must understand to master this topic and answer exam questions confidently.

What Is Metabolism?
Metabolism includes all chemical reactions occurring in cells. It maintains life through two opposite but complementary processes:
- Catabolism – breakdown of complex molecules into simpler ones; releases energy.
- Anabolism – synthesis of complex molecules from simpler ones; requires energy.
Comparison Table: Catabolism vs. Anabolism
| Feature | Catabolism | Anabolism |
| Reaction Type | Exergonic (energy-releasing) | Endergonic (energy-requiring) |
| Function | Breaks down molecules | Builds molecules |
| Example | Glucose → CO₂ + H₂O (respiration) | Amino acids → Proteins |
| ATP Role | Produces ATP | Consumes ATP |
Catabolic reactions provide the energy used to fuel anabolic reactions. This relationship is vital for maintaining cellular energy balance.
Thermodynamics in Metabolism
Biological systems follow the laws of thermodynamics, which dictate how energy behaves in reactions.
1. First Law of Thermodynamics
Energy cannot be created or destroyed. It can only change form. Cells convert food into ATP, but don't create energy from nothing.
2. Second Law of Thermodynamics
Every energy transformation increases the universe's entropy (disorder). In cells, not all energy from food becomes usable-some is lost as heat.
3. Gibbs Free Energy (ΔG)
Free energy predicts whether a reaction will occur spontaneously.
| ΔG Value | Reaction Type | Description |
| ΔG < 0 | Exergonic | Releases energy; spontaneous |
| ΔG > 0 | Endergonic | Requires energy; non-spontaneous |
| ΔG = 0 | Equilibrium | No net change |
Cells avoid equilibrium because it implies no energy flow-equilibrium means death in biological systems.
ATP: The Energy Currency
ATP (adenosine triphosphate) powers nearly every cellular activity. It consists of adenine, ribose, and three phosphate groups. Breaking one phosphate bond (ATP → ADP + Pi) releases energy.
ATP Hydrolysis
- Exergonic: Releases ~7.3 kcal/mol.
- Drives endergonic processes like muscle contraction or protein synthesis.
Energy Coupling
Cells use ATP to couple an exergonic reaction (ATP hydrolysis) with an endergonic one, making the combined reaction favorable.
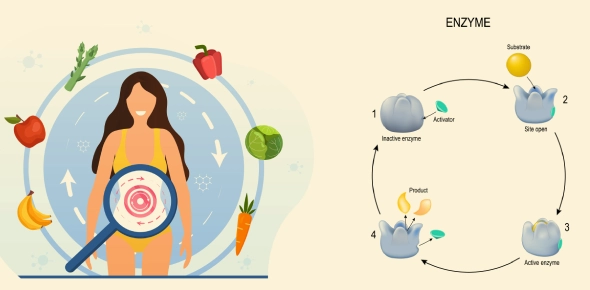
Enzymes: Biological Catalysts
Enzymes are proteins that accelerate chemical reactions by lowering the activation energy (Ea).
Key Characteristics
- Highly specific: Each enzyme acts on a specific substrate.
- Reusable: Not consumed in reactions.
- Do not change ΔG: Only affect reaction rate, not energy balance.
Enzyme-Substrate Complex
Enzymes have an active site where substrates bind. The induced fit model explains how enzymes adjust shape slightly to fit substrates better.
Activation Energy Explained
Even spontaneous reactions need a "push" to begin-this is the activation energy. Enzymes lower this barrier.
| Reaction Type | Activation Energy | Speed Without Enzyme | With Enzyme |
| Exergonic | High | Slow | Fast |
| Enzyme Effect | Lowers Ea | Faster reaction |
Without enzymes, most biological reactions would occur too slowly to sustain life.
Factors Affecting Enzyme Activity
1. Temperature
- Too low: reactions slow down.
- Too high: enzyme denatures (loses shape).
2. pH
- Each enzyme has an optimal pH range.
- Outside this range: activity drops sharply.
3. Substrate Concentration
- Increased substrate = faster reaction, up to saturation point.
Enzyme Inhibition and Regulation
Enzymes are regulated to maintain homeostasis and efficiency.
Types of Inhibition
| Type | Binding Site | Effect | Reversible? | Overcome by [S]? |
| Competitive | Active site | Blocks substrate | Yes | Yes |
| Noncompetitive | Allosteric site | Changes enzyme shape | Yes | No |
| Feedback Inhibition | Allosteric site | Inhibits pathway from end | Yes | N/A |
Feedback Inhibition
This is when the end product of a pathway inhibits the enzyme that catalyzes the first step. It ensures that the cell stops producing products it already has in sufficient quantity.
Example
Pathway: A → B → C → D → E (final product)
If E is abundant, it inhibits the enzyme converting A to B.
Role of Cofactors and Coenzymes
Enzymes sometimes require helper molecules:
| Helper Type | Description | Example |
| Cofactor | Inorganic (metal ions) | Zn²⁺, Fe²⁺ |
| Coenzyme | Organic (often vitamin-derived) | NAD⁺, FAD |
Without these helpers, some enzymes cannot function.
Allosteric Regulation
Enzymes can have regulatory sites (not the active site) that bind activators or inhibitors, changing their activity.
- Allosteric Inhibitor: Reduces activity.
- Allosteric Activator: Enhances activity.
- Seen in enzymes with multiple subunits (e.g., hemoglobin, though not an enzyme).
Sample Concept Checks
Q1. What term describes breaking down large molecules?
- Answer: Catabolism.
Q2. Which energy law states that entropy increases?
- Answer: Second Law of Thermodynamics.
Q3. ATP is used to couple what type of reactions?
- Answer: Endergonic reactions.
Q4. What does ΔG = 0 mean?
- Answer: Reaction is at equilibrium.
Q5. How do enzymes accelerate reactions?
- Answer: By lowering activation energy.
Enzyme Graph: With vs. Without Enzyme
A reaction energy diagram would show:
- Higher peak (Ea) without enzyme.
- Lower peak (Ea) with enzyme.
- Start and end points (ΔG) remain the same in both.
Misconceptions to Avoid
- Enzymes do not provide energy.
- Enzymes do not shift equilibrium.
- ΔG tells if a reaction is spontaneous, not how fast it occurs.
- Not all inhibition is permanent; many inhibitors are reversible.
Core Concepts
| Topic | Key Insight |
| Metabolism | Sum of all chemical reactions |
| Catabolism | Breaks down molecules; releases energy |
| Anabolism | Builds molecules; requires energy |
| Thermodynamics | Energy transformations follow physical laws |
| ATP | Universal energy carrier |
| Enzymes | Speed up reactions by lowering Ea |
| Active Site | Where substrate binds and reaction occurs |
| Activation Energy | Energy needed to start a reaction |
| Inhibition | Competitive or noncompetitive ways to regulate |
| Feedback Inhibition | Product inhibits pathway start point |
| Cofactors & Coenzymes | Required enzyme helpers |
| Allosteric Sites | Regulatory locations on enzyme |
Key Takeaway
Mastering metabolism and enzymes equips students to tackle core biology topics like respiration, biosynthesis, and thermoregulation. The key is understanding how energy is stored, transferred, and regulated-and how enzymes enable life to happen quickly and efficiently. Revisit this guide alongside practice quizzes to deepen your retention.
Take This Quiz
Rate this lesson:
 Back to top
Back to top