|
Polymer |
|
A long molecule consisting of many similar or identical monomers linked together by covalent bonds |
| |
|
Monomer |
|
The subunit that serves as the building block of a polymer |
| |
|
Enzymes |
|
Protein catalysts |
| |
|
Tuber |
|
A short, thick, underground plant stem (e.x. the white potato) |
| |
|
Atherosclerosis |
|
A cardiovascular disease in which fatty deposits develop on the inner walls of the arteries, obstructing the arteries and causing them to harden. |
| |
|
Essential Fatty Acid |
|
An unsaturated fatty acid that an animal needs but cannot make |
| |
|
Catalysts |
|
A chemical agent that selectively increases the rate of a reaction without being consumed by the reaction |
| |
|
Peptide Bond |
|
The covalent bond between the carboxyl group on one amino acid and the amino group on another, formed by a dehydration reaction |
| |
|
Denaturation of protein |
|
Disruption of the weak internal chemical bonds and interactions causes the protein to lose its native shape |
| |
|
Gene |
|
A discrete unit of hereditary information consisting of a specific nucleotide sequence |
| |
|
Which of the four main classes of important large molecules of life does not consist of polymers? |
|
Lipids |
| |
|
What is a dehydration reaction? |
|
A reaction in which 2 molecules become covalently bonded to each other with the removal of a water molecule |
| |
|
Why is hydrolysis the reverse of the dehydration reaction? |
|
Because it breaks the bonds betweeonomers by adding water molecules. (Hydrogen attaches to one monmer and the hydroxyl group to another) |
| |
|
What is the most common monosaccharide? |
|
Glucose |
| |
|
What type of isomers are glucose and fructose? |
|
Structural isomers |
| |
|
Glucose is an aldose while fructose is a ketose. If they are isomers, how do they differ? |
|
Different location of a carbonyl group |
| |
|
Plants synthesize sucrose in their leaves. What happens to this carbohydrate? |
|
They transport it from their leaves to roots and other nonphotosynthetic organs in the form of sucrose |
| |
|
What are the three main grasses that provide starch for humans? |
|
Wheat, rice, and corn |
| |
|
In our body, where is glycogen stored? |
|
Mainly in the liver and muscle cells |
| |
|
What is the most abundant organic compound on Earth? |
|
Cellulose |
| |
|
Explain how the glycosidic linkages differ between starch and cellulose |
|
There are 2 different ring structures for glucose. When GLUCOSE form a ring, the hydroxyl group attached to the #1 carbon is positioned above or below the plane of the ring. 2 ring forms are called alpha and beta. In STARCH, all the glucose monomers are in the alpha configuration. |
| |
|
What is the main difference in molecular shape between starch and cellulose? |
|
Starch molecules are mostly helical, and cellulose molecules are straight |
| |
|
What is the reason that enzymes that can digest starch cannot digest cellulose? |
|
Enzymes digest starch by hydrolizing alpha linkages but not beta linkages because of their different shape |
| |
|
If cows cannot digest cellulose, how can they obtain nutritive value from eating hay/grass? |
|
They have cellulose digesting prokaryotes and protists in their stomach. Microbes hydrolize cellulose of hay and frass and convert glucose to other compounds which nourish the cow. |
| |
|
Draw and label the structure of glycerol |
|
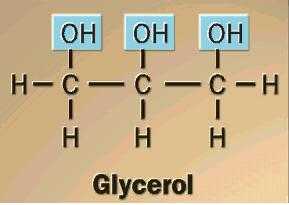
|
| |
|
What concern does your textbook have regarding a diet rich in saturated fats? |
|
Contributes to atherosclerosis (Plaque develops in blood vessels, reducing flow and resilience of vessels) |
| |
|
How is a phospholipid different from a fat? |
|
Only has 2 fatty acids attached to the glycerol rather than 3 |
| |
|
What is an important role of cholesterol in the body? |
|
Is is the molecule from which other steroids (such as sex hormone), are synthesized. It is also a common component of animal cell membranes. |
| |
|
Draw and label the structure of an amino acid |
|

|
| |
|
Transthyretin (TTR) is a protein fouin serum and cerebrospinal fluid. How many amino acids are used to make the entire protein? |
|
It is made of 4 identical polypeptide chains, each with 127 amino acids. (508) |
| |
|
Transthyretin transports thyroxine (T4- a thyroid hormone) and retinol (vitamin A), hence its name: transports thyroxine and retinol. What determines the precise primary structure of TTR? |
|
Inherited genetic information |
| |
|
TTR is a 62-kDa homotetramer that is synthesized in the liver, choroid plexus and retinal pigment epithelium. What is the cause of its secondary structure? |
|
The cause of secondary structure is dictated by primary structure. Result of H bonds between repeating constituents of the polypeptide backbone |
| |
|
TTR is rich in beta sheet structure. What is the other type of secondary structure available for polypeptides (and found in TTR)? |
|
Alpha helix (delicate coild held together by H bonding between every 4th amino acid) |
| |
|
What are the 5 different types of interactions that shape the TTR polypeptide. What are they? |
|
1)Hydrogen bond
2) Disulfide Bridge
3) Polypeptide backbone
4) Hydrophobic interactions and van der waals interactions
5) Ionic bond |
| |
|
What gives collagen its great strength? |
|
It is a fibrous protein with 3 identical helical polypeptides intertwined into a triple helix |
| |
|
Describe the quaternary structure of hemoglobin |
|
Has 2 alpha particles and 2 beta particles. Molecules don't associate with each other, and each carries O2 |
| |
|
How many amino acids in one Beta chain are changed to cause sickle-cell disease? |
|
1 |
| |
|
What is it about protein structure that explains why excessively high fevers can be fatal? |
|
High temperature denatures a protein, causing it to be biologically inactive. Proteins in blood can denature at high temperatures. |
| |
|
Where in the cytoplasm are polypeptides synthesized? |
|
ribosome |
| |
|
Which (type of) molecule conveys genetic instructions from the nucleus to the cytoplasm? |
|
MRNA |
| |
|
If you were given a sequence of nitrogenous bases, how could you determine whether you were looking at DNA or at RNA? |
|
-Thymine is fund in only DNA and uracil is only found in RNA
-In DNA, sugar is deoxyribose and RNA it is ribose. (lacks oxygen on second carbon in ring) |
| |
|
How did deoxyribose get its name? |
|
Deoxyribose got its name because deoxyribose lacks an oxygen atom on the second carbon in the ring. |
| |
|
How do we distinguish the numbers of the sugar carbons from those used for the ring atoms to attached nitrogenous base? |
|
We can distinguish it because sugar carbon numbers of a nucleoside/nucleotide have a (1) after them (2^1 carbon, 5^1 carbon) |
| |
|
What holds the two strands of DNA together? |
|
Hydrogen bonds between paired bases |
| |
|
What accounts for each of the daughter cells (after cellular division) being genetically identical to the parent cell? |
|
DNA's 2 strands are complementary. When cell divides, copies are distributed to daughter cell, making them identical to parent cell. |
| |
|
What is the role of tRNA? |
|
Brings amino acids to the ribosome during the synthesis of a polypeptide |
| |
|
The architecture of a large biological molecule helps explain how that molecule (blank) |
|
works |
| |
|
The molecular logic od life is simple but elegant: Small (blank) common to all organisms are ordered into unique (blank) |
|
molecules, macro-molecules) |
| |
|
Carbohydrates include both sugars and (blank) of sugar |
|
polymers |
| |
|
Most names for sugars end in - (blank) |
|
ose |
| |
|
Sugars that have 6 carbons are called (blank) |
|
hexoses |
| |
|
Sugars that have 5 carbons are called (blank) |
|
pentoses |
| |
|
Sugars that haved 3 carbons are called (blank) |
|
trioses |
| |
|
A disaccharide consists of 2 (blank) joined by a glycosidic linkage |
|
monosaccharides |
| |
|
The monomers comprising sucrose are (blank) and (blank) |
|
glucose, fructose |
| |
|
Polysaccharides are macromolecules, polymers with a few hundred to a few thousand (blank) joined by glycosidic linkages |
|
monosaccharides |
| |
|
Plants store sucrose in the form of (blank), a polymer of (blank) monomers |
|
starch, glucose |
| |
|
Potato tubers and grains are foods rich in (blank) |
|
starch |
| |
|
Plants store glucose as starch; vertebrates store glucose as (blank) |
|
glycogen |
| |
|
A cellulose molecule is an unbranched (blank)-glucose polymer |
|
beta |
| |
|
The exoskeleton of arthropods is composed of (blank) |
|
chitin |
| |
|
The chemical formula for glucose is C(blank)H(blank)O(blank) |
|
6, 12, 6 |
| |
|
A fat is constructed from two kinds of smaller molecules: (blank) and (blankX2) |
|
glycerol, fatty acids |
| |
|
Another name for a fat is triaglycerol. Still another name is (blank) |
|
triglyceride |
| |
|
A fat made from saturated fatty acids, is called a (blank) fat |
|
saturated |
| |
|
Saturated animal fats are (blank) at room temperature |
|
solid |
| |
|
Unsaturated plant fats are (blank) at room temperature |
|
liquid |
| |
|
The major function of fats is (blankX2) |
|
energy storage |
| |
|
The hydrocarbon tails of a phospholipid are (blank) toward water |
|
hydrophobic |
| |
|
The phospholipid bilayer forms a (blank) between the cell and its external environment |
|
boundary |
| |
|
In vertebrates, cholesterol is synthesized in the (blank) and obtained from the (blank) |
|
liver, diet |
| |
|
A high level of cholesterol in the blood may contribute to (blank) |
|
atherosclerosis |
| |
|
All proteins are constructed from a set of (blank) amino acids |
|
20 |
| |
|
Polymers of amino acids are called (blank) |
|
polypeptides |
| |
|
Casein, the protein of milk, is the major source of amino acids for baby (blank) |
|
mammals |
| |
|
Ovalbumin is the protein of egg white, used as an amino acid source for the developing avian (blank) |
|
embryo |
| |
|
Hemoglobin transports (blank) |
|
oxygen |
| |
|
The proteins actin and myosin are responsible (along with other molecules) for the contraction of (blank) |
|
muscles |
| |
|
The chemical nature of the protein molecule as a whole is determined by the kind and sequence of the (blankX2) of the amino acids |
|
side chains |
| |
|
It is the amino acid sequence of each polypeptide that determines what three-dimensional structure the (blank) will have under normal cellular conditions |
|
protein |
| |
|
A protein's specific structure determines how it (blank) |
|
works |
| |
|
Within the backbone of the repeating constituents of a polypeptide, the oxygen atoms have a partial negative charge, and the hydrogen atoms attached to the nitrogens have a partial positive charge, therefore (blank) bonds can form between these atoms |
|
hydrogen |
| |
|
Some fibrous proteins, such as alpha-keratin, the structural protein of hair, have the (blank) formation over most of their length |
|
alpha helix |
| |
|
(blankX2) form when two cyteine monomer, which have sulfhydryl groups (-SH) on their side chains, are brought together by the folding of the polypeptide |
|
Disulfide bridges |
| |
|
The complete globular transthyretin protein is made up of (blank) polypeptides |
|
4 |
| |
|
The complete globular transthyretin protein is made up of (blank) polypeptides |
|
4 |
| |
|
The complete globular hemoglobin protein is made up of (blank) polypeptides |
|
4 |
| |
|
Each polypeptide of hemoglobin has a component, called heme, with an iron atom that binds (blank) |
|
oxygen |
| |
|
Crucial to the folding process are (blank), protein molecules that assist the proper folding of other proteins |
|
chaperonins |
| |
|
Nucleic acids are polymers made of monomers called (blank) |
|
nucleotides |
| |
|
Unique among molecules, (blank) provides direction for its own replication |
|
DNA |
| |
|
DNA also directs (blank) synthesis and, through (blank), controls protein synthesis |
|
RNA, RNA |
| |
|
Each chromosome contains one long (blank) molecule, usually carrying several hundred or more genes |
|
DNA |
| |
|
The portion of a nucleotide without any phosphate groups is caclled a (blank) |
|
nucleoside |
| |
|
There are 2 families of nitrogenous bases: (blank) and (blank) |
|
pyrimidines, purines |
| |
|
The sequence of bases along a DNA or mRNA polymer is (blank) for each gene |
|
unique |
| |
|
In DNA, adenine always pairs with (blank) and guanine always pairs with (blank) |
|
thymine, cytosine |
| |
|
The four main classes of large biological molecules |
|
1) carbohydrates
2) proteins
3) nucleic acids
4) lipids |
| |
|
4 diseases that are the result of misfolded proteins |
|
1) Parkinson's
2) Alzheimer's
3) Huntington's
4) Cancer |
| |
|
Diagram and Label the Structure and function of a phospholipid |
|

|
| |
|
The structure formed when phospholipids self assemble |
|

|
| |
|
The flow of genetic information |
|

|
| |
|
Explain how digestion (within our bodies) illustrates hydrolysis |
|
The bulk of the organic material in our food is in the form of polymers that are much too large to enter our cells. Within the difestive tract, various enzymes attack the polymers, speeding up hydrolysis, THe released monomers are then absorbed into the bloodsttream for distribution to all body cells. Those cells can then use dehydration reactions to assemble the monomers into new, different polymers that can perform specific functions required by the cell. |
| |
|
Describe the four levels of protein structure |
|
-
Primary structure: the linear arrangment of amino acids in a protein and the location of covalent linkages such as disulfide bonds between amino acids.
-
Secondary structure: areas of folding or coiling within a protein; examples include alpha helices and pleated sheets, which are stabilized by hydrogen bonding.
-
Tertiary structure: the final three-dimensional structure of a protein, which results from a large number of non-covalent interactions between amino acids.
-
Quaternary structure: non-covalent interactions that bind multiple polypeptides into a single, larger protein. Hemoglobin has quaternary structure due to association of two alpha globin and two beta globin polyproteins.
|
| |
