|
Rows are called |
|
Rows are called periods |
| |
|
Columns are called |
|
Columns are called groups or families |
| |
|
General Info on Noble Gases |
|
- Group 18 (8A)-Monatomic-Noble, content with being a single atom and rarely do they bond with other atoms or each other-Can be liquified by decreasing temp &/or increasing pressure |
| |
|
General Info on Alkali Metals |
|
-Group 1 (1A)-Soft, shiny metals-React w/ many substances including the water vapor in air-Tend to LOSE an E- therefore they tend to be POSITIVE ions (+1) |
| |
|
General Info on Halogens |
|
-Group 17 (7A)-Neutral at room temp-Stable only when diatomic (F2)-Very reactive -Tend to GAIN an E- making it a NEGATIVE ion(-1)-Haloid Ion: A single halogen atom with a -1 charge |
| |
|
General Info on Hydrogen |
|
-Group 1 (1A)-Has the characteristics of both alkali metals and halogens |
| |
Staircase on the Periodic Table starts at ? and goes in which direction?
What does it signify? |
|
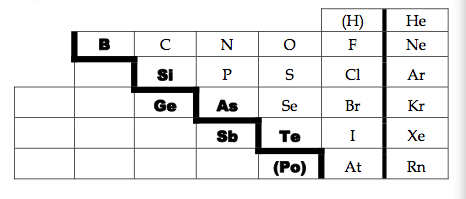
The staircase on the Periodic Table starts at B and runs diagonally down to the right.
It signifies the separation of metal and nonmetal elements. |
| |
|
What are the elements which border the staircase (excluding Al)? |
|
The 6 elements bordering the staircase, which is not including Al, are the metalloids.
B, Si, Ge, As, Sb, Te, (Po) - sometimes Po is considered it, depends on textbook |
| |
|
What has a chemical behavior that is in-between that of metals and nonmetals? |
|
Metalloids have a chemical behavior that is in-between metals and nonmetals. |
| |
Where are Nonmetals located on the periodic table?
How many nonmetals are there? |
|
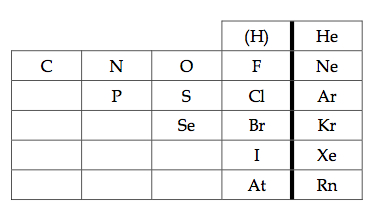
Nonmetals are located to the right of the metalloids on the periodic table.
There are a total of 18 nonmetals |
| |
|
What is Hydrogen?A metal, nonmetal, or metalloid? Even though it is in group 1? |
|
Hydrogen, even though it is placed in group one, is considered to be a nonmetal.
Hydrogen has unique properties but mostly acts like a nonmetal. |
| |
|
Where are the metals on the periodic table? |
|
The metals are found to the left of the metalloids on the periodic table. |
| |
|
Family that rarely bonds to other atoms is |
|
Noble Gases
|
| |
|
Lightest nonmetal |
|
Hydrogen |
| |
|
Lightest metalloid |
|
Boron (B) |
| |
|
Tend to form -1 ions |
|
Ions formed by halogen atoms |
| |
|
Family forms +1 ions |
|
Ions formed by alkali metals |
| |
|
Family forms +2 ions |
|
Ions formed by column 2 atoms |
| |
|
Name for halogen atoms with a -1 charge |
|
Halide |
| |
|
Symbols for the 5 lightest alkali metal atoms |
|
Li, Na, K, Rb, Cs |
| |
|
Symbols for the 5 lightest column 2 atoms |
|
Be, Mg, Ca, Sr, Ba |
| |
|
Symbols for the 5 halogen atoms |
|
F, Cl, Br, I, At
Memory Link:
LightbulbLightbulb (represents Halogen)... a fox runs around the inside of the bulb, a giant club smashes the bulb and kills the fox, a huge broom cleans up the dead fox and the broken glass, and sweeps it all into an igloo. Then a giant atom crashes out of sky squishing the igloo. |
| |
|
Symbols for the 12 nonmetals that tend to bond |
|
H, C, N, O, P, S, Se plus the 5 Halogens (F, Cl, Br, I, At)
Memory Link:
The symbol used for nonmetal in linking is: a large metal block with a giant red "X" crossing it out so make 2 of them bonded together to represent nonmetals that bond. This crossed out block of metal falls down on a cat wearing a hat. The cat falls on a nail that impales him. Blood squirts everywhere and an octopus cleans up the blood quickly with its 8 arms. A penguin sneaks around from behind the octopus and cuts off its arms with a giant pair of scissors. A seagull flies overhead and shits down on the scissors making them rusty then crashes into a crucified (plus sign) lightbulb (symbol for Halogens). |
| |
|
Copper |
|
Cu |
| |
|
Tin |
|
Sn |
| |
|
Mercury |
|
Hg |
| |
|
Gold |
|
Au |
| |
|
Potassium |
|
K |
| |
|
Iron |
|
Fe |
| |
|
Lead |
|
Pb |
| |
|
Silver |
|
Ag |
| |
