Resume Quiz
Lewis Electron Dot Diagram
2 Kesyon
5 Minit
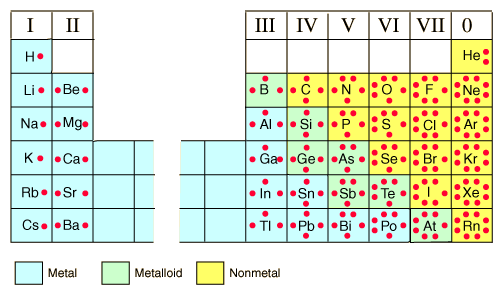 A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy.
A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy.