Related Flashcards
Related Topics
Cards In This Set
| Front | Back |
|
Add a branched group
|
Tag a Br on the end that adds to ring, use AlCl3 as catalyst
|
|
Add a halide
-Cl
|
FeCl3
Cl-Cl
|
|
Add NO2
|
HNO3
H2SO4
|
|
Convert NO2 to NH2
|
ZnHg & HCl
or
H2Pd
|
|
Convert NH2 to NO2
|
Oxidize
CR3COOOH
|
|
Reduce something with a double bond to oxygen to a single-bond hydrocarbon
|
ZnHg & HCl
or
H2Pd
|
|
Oxidize a hydrocarbon to contain a double bonded oxygen
|
CrO3
H2SO4
H2O
|
|
Oxidize an alkyl
|
KMnO4
|
|
Total reduction (including armomaticity)
|
H2Pt at 2000psi
or
H2Rh
|
|
Add SO3
|
SO3
H2SO4
|
|
Remove SO3
|
H3O+
H2O
|
|
Grignard Reaction
|
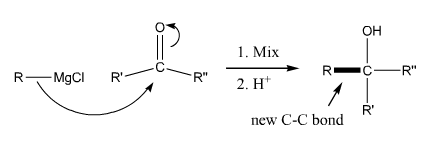 Grignard reaction. This reaction adds a halomagnesium reagent (Grignard reagent) to a carbonyl to make an alcohol. |
|
Diels-Alder
|
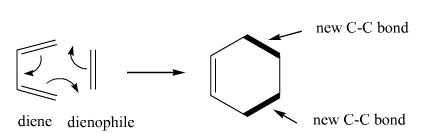 Diels-Alder reaction. This reaction takes a diene and a dienophile to make ringed and bicyclic products. Makes 2 C-C bonds. |
|
Friedel-Crafts
|
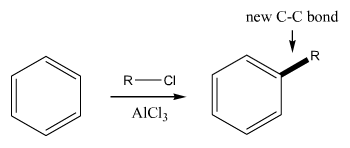 Friedel-Crafts reactions (for aromatic rings). This reaction makes an aromatic-carbon bond. |
|
Wittig
|
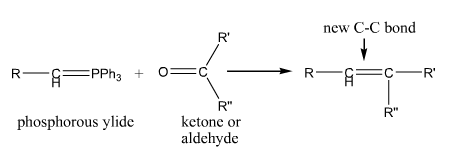 The Wittig Reaction. Makes a carbon-carbon double bond starting with a carbonyl compound phosphonium ylide |





