Related Flashcards
Related Topics
Cards In This Set
| Front | Back |
|
19.1.1:
Describe the standard hydrogen
electrode.
|
Standard hydrogen electrode: The half cell containing hydrogen gas at 1atm being bubbled through a
H+ (ie HCl) solution. – note all at standard conditions.
The Standard Hydrogen Electrode (SHE) is assigned a value of 0 V under standard conditions of 25°C, solution concentrations of 1.0 mol dm−3 and 1 atmosphere pressure for gases. Positive E°cell indicates a spontaneous reaction (– ΔG°); negative E°cell means a non- spontaneous in the forward direction, or a spontaneous reverse reaction. |
|
19.1.2:
Define the term standard electrode
potential (E Ö ) .
|
Standard electrode potential ( Eφ ): The potential difference between a given half-cell and the
standard hydrogen electrode.
Standard electrode/reduction potential is the e.m.f. of a half-cell connected to the standard hydrogen potential (at 25°C, 1.0 mol dm−3 solution concentrations and gas at 1 atmosphere pressure). If reduction occurs at the standard half-cell, its sign is +; its sign is negative if reduction occurs at the standard hydrogen electrode (see Table 15). |
|
19.1.4:
Predict whether a reaction will
be spontaneous using standard
electrode potential values.
|
Positive E°cell indicates a spontaneous reaction (– ΔG°); negative E°cell means a non-
spontaneous in the forward direction, or a spontaneous reverse reaction.
|
|
19.2.1:
Predict and explain the products of
electrolysis of aqueous solutions.
|
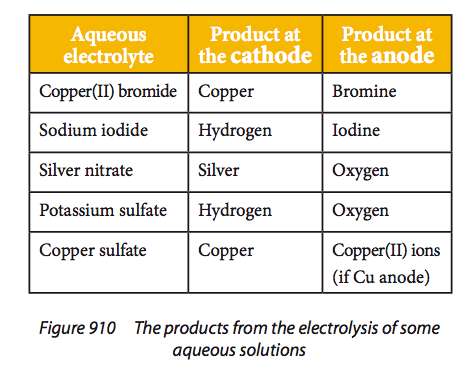 Explanations should refer to EÖ values, nature of the electrode and concentration of the electrolyte. Examples include the electrolysis of water, aqueous sodium chloride and aqueous copper(II) sulfate. When considering the products from the electrolysis of an aqueous solution the possible reactions: Cathode - formation of the metal or formation of hydrogen, Anode - formation of the non-metal, formation of oxygen or oxidation of the electrode, must be considered and the most energetically favourable will take place. For the cathode this will be the one with the most positive; for the anode the one with the most negative electrode potential. For most common electrolytes: Cathode - hydrogen is formed unless Cu2+ or Ag+ are present, Anode - oxygen is formed unless Br– or I– are present. |
|
19.2.2: Determine the relative amounts of the
products formed during electrolysis.
|
The factors to be considered are charge on the ion,
current and duration of electrolysis.
The amount of product that results from electrolysis will depend upon:
|
|
19.2.3:
Describe the use of electrolysis in
electroplating.
|
His process is used to purify copper
for use as an electrical conductor. The cathode becomes
coated in a layer of the metal, a process called electroplating.
Similarly electrolysis of a solution containing silver ions
(or the ions of some other easily reduced metal) will result
in the metal being deposited on the cathode and this
process is often used to electroplate less reactive metals
on to cathodes made of more reactive metals, either for
decoration or protection against corrosion.
|