Related Flashcards
Related Topics
Cards In This Set
| Front | Back |
|
16.1.1:
Distinguish between the terms rate
constant, overall order of reaction and
order of reaction with respect to a
particular reactant.
|
Rate expression: The concentrations of the reactants to the power of the order of each product
multiplied by the rate constant: Rate=k[A]m[B]n[C]o
Order of reaction: The order of reaction is said to be m for substance A, n for substance B, etc. The
total order of reaction is the sum of these powers, ie m + n etc = overall order
Rate constant: The constant in the above equation represents the proportionality between the reaction
rate and the concentrations of the reactants. Its units depend on the number of reactants and their
orders.
|
|
16.1.2:
Deduce the rate expression for a
reaction from experimental data.
|
It is an experimentally determined equation in that the information (n,m,k etc) can only be found through experimentation and not through theoretical considerations. The rate equation shows the relationship between the speed of a reaction and the concentrations of the individual reactants. Once the orders are found then they provide information regarding the mechanism of the specific reaction.
|
|
16.1.4:
Sketch, identify and analyse graphical
representations for zero-, first- and
second-order reactions.
|
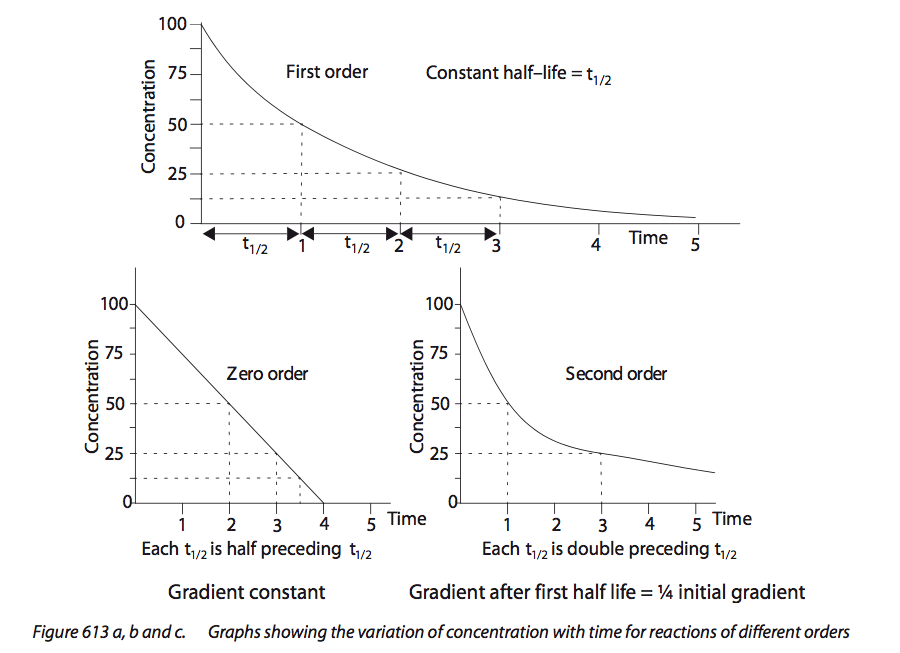 Students should be familiar with both concentration–time and rate–concentration graphs. |
|
16.2.1:
Explain that reactions can occur by
more than one step and that the
slowest step determines the rate of
reaction (rate-determining step).
|
Rate determining step: The slowest step in the sequence of steps leading to the formation of products. Molecularity: The number of molecules reacting in an elementary step.
|
|
16.2.2:
Describe the relationship between
reaction mechanism, order of reaction
and rate-determining step.
|
Only examples with one- or two-step reactions
where the mechanism is given will be assessed.
In summary, a mechanism is a model of how a reaction occurs. The rate of overall reaction is the rate of the slowest step. This slowest step is called the rate determining step. Species produced in earlier steps of the mechanism that are consumed in later steps are called intermediates. A mechanism must account for the overall stoichiometry of the reaction, the observed rate expression and any other available evidence (such as the effect of light or a catalyst). |
|
16.3.1:
Describe qualitatively the relationship
between the rate constant (k) and
temperature (T).
|
Arrhenius Eqn:
k=Ae− Ea/RT Rate increases with temperature when concentrations are constant, k increases rapidly with T and is temperature dependent, producing a straight line graph of ln k vs 1/T with a negative slope. Slope of the line = - Ea/R. Thus Ea can be determined; T must be in Kelvin, not °C. K is y intercept. Arrhenius constant is the collision factor that represents the frequency of successful collisions with the favourable geometry, R is the Ideal Gas Constant (= 8.314 J mol-1 K- 1), Ea is the activation energy (the minimum energy required for a reaction to occur), and T must be in Kelvin scale. |
|
16.3.2:
Determine activation energy (Ea)
values from the Arrhenius equation
by a graphical method.
|
The Arrhenius equation and its logarithmic form are
provided in the Chemistry data booklet. The use of
simultaneous equations will not be assessed.
|