Related Flashcards
Related Topics
Cards In This Set
| Front | Back |
|
12.1.1:
Explain how evidence from first
ionization energies across periods
accounts for the existence of main
energy levels and sub-levels in atoms.
|
Ionisation energy: The energy that is required (or given out) to overcome the attraction of the nuclear
charge and remove an electron from a gaseous atom. Removing 1 electron results in a +1 charge.
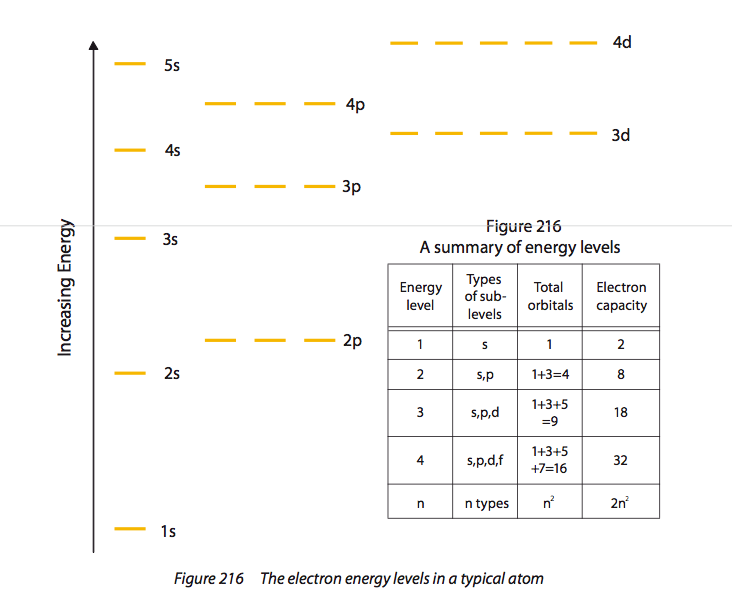
Main (or principal) energy levels, sub-levels and orbitals: The main energy levels, n are assigned whole number integers, n = 1, 2, 3, 4... . n = 1 represents the lowest energy level. Each main energy level contains n sub-levels and a total of n^2 orbitals. The magnitude of the ionisation energy will depend on the charge on the nucleus. The more electrons that have been removed from an atom, the greater the energy required to remove the next electron. When the successive electrons are all in the same energy level this is because of a reduction in the amount of electron-electron repulsion and hence the greater nuclear-electron attraction that results causes the remaining electrons to move closer to the nucleus. |
|
12.1.2:
Explain how successive ionization
energy data is related to the electron
configuration of an atom.
|
Sometimes with successive ionisation energies the next
electron must be removed from a filled inner energy level,
so that this electron will experience a much higher effective
nuclear charge and there is a sudden large
rise in ionisation energy.
There is a steady increase in successive ionization energies for every element (decrease in e−-e− repulsion) and a steady increase going across the period (increase in nuclear charge). |
|
12.1.3:
State the relative energies of s, p, d
and f orbitals in a single energy level.
|
State. s
|
|
12.1.4:
State the maximum number of
orbitals in a given energy level.
|
Orbital: The cloud shapes that electron pairs travel around the nucleus in the quantum mechanical
model.
Each energy sub-level is divided into orbitals each of which can contain up to two electrons, which must have opposite spins, as a consequence of the Pauli exclusion principle, which says that no two electrons in an atom can be in exactly the same state. Each orbital can have a maximum of 2 electrons. n = 1 has one sub-level which is called an s sub-level and which contains one s orbital. n = 2 has two sub-levels: 2s and 2p; n = 3 has 3 sub-levels: 3s, 3p and 3d; n = 4 has 4 sub-levels:4s, 4p, 4d and 4f, etc. |
|
12.1.5:
Draw the shape of an s orbital and the
shapes of the px, py and pz orbitals.
|
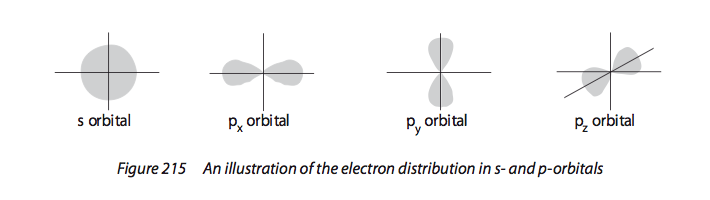 See image. |
|
12.1.6:
Apply the Aufbau principle, Hund’s
rule and the Pauli exclusion principle
to write electron configurations for
atoms and ions up to Z = 54.
|
 For Z = 23, the full electron configuration is 1s22s22p63s23p64s23d3 and the abbreviated electron configuration is [Ar]4s23d3 or [Ar]3d34s2. Exceptions to the principle for copper and chromium should be known. Students should be familiar with the representation of the spinning electron in an orbital as an arrow in a box. Aufbau principle: Electrons enter orbitals of lowest energy first. Pauli exclusion principle: An atomic orbital may describe at most two electrons. Hunds rule: When electrons occupy orbitals of equal energy, one electron enters each orbital until all the orbitals contain one electron with spins parallel. |