|
Add a branched group |
|
Tag a Br on the end that adds to ring, use AlCl3 as catalyst |
| |
|
Add a halide
-Cl |
|
FeCl3
Cl-Cl |
| |
|
Add NO2 |
|
HNO3
H2SO4 |
| |
|
Convert NO2 to NH2 |
|
ZnHg & HCl
or
H2Pd |
| |
|
Convert NH2 to NO2 |
|
oxidize
CR3COOOH |
| |
|
Reduce something with a double bond to oxygen to a single-bond hydrocarbon |
|
ZnHg & HCl
or
H2Pd |
| |
|
Oxidize a hydrocarbon to contain a double bonded oxygen |
|
CrO3
H2SO4
H2O |
| |
|
Oxidize an alkyl |
|
KMnO4 |
| |
|
Total reduction (including armomaticity) |
|
H2Pt at 2000psi
or
H2Rh |
| |
|
Add SO3 |
|
SO3
H2SO4 |
| |
|
Remove SO3 |
|
H3O+
H2O |
| |
|
Grignard Reaction |
|
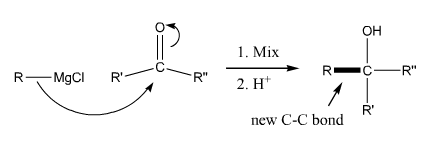
Grignard reaction. This reaction adds a halomagnesium reagent (Grignard reagent) to a carbonyl to make an alcohol. |
| |
|
Diels-Alder |
|
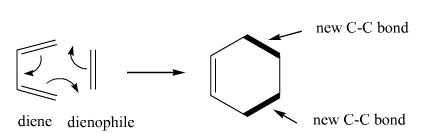
Diels-Alder reaction. This reaction takes a diene and a dienophile to make ringed and bicyclic products. Makes 2 C-C bonds. |
| |
|
Friedel-Crafts |
|
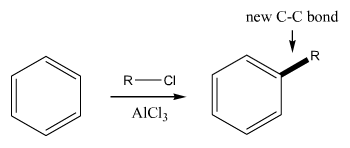
Friedel-Crafts reactions (for aromatic rings). This reaction makes an aromatic-carbon bond. |
| |
|
Wittig |
|
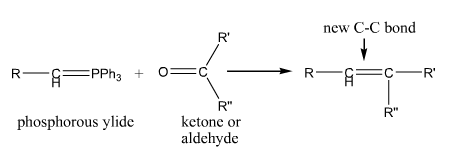
The Wittig Reaction. Makes a carbon-carbon double bond starting with a carbonyl compound phosphonium ylide |
| |
|
Cyanide addition |
|
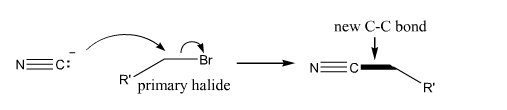
Cyanide additions to primary halides. |
| |
|
Acetylide Reactions |
|
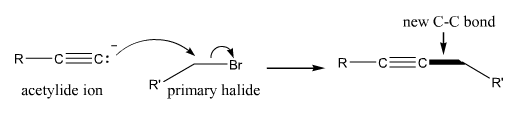
Acetylide reactions. These reactions involve use of an acetylide (a deprotonated terminal alkyne) as a nucleophile. Typically, the acetylide is used to attack a primary halide (or a carbonyl group to make an alcohol. |
| |
